Summary Points
-The testes have two main functions of spermatogenesis, or production of sperm (an endocrine function), and androgens male (hormonal secretion), its exocrine function.
-Trauma to the testis that may cause torsion is important because ischemia of only 1-3 hours, for example, results in decreased spermatogenesis and irreversible changes occur in only 6-8 hours.
-Testicular cancer is the most commonly occurring malignancy in men between the ages of 15 and 35. The incidence for mixed germ cell tumors alone is two to three cases per 100,000 males per year. Testicular cancer makes up about 1 percent of all cancers in men in the United States.
-The male reproductive system develops embryonically under the influence of the “Y” chromosome, testosterone, and female inhibitory substances. The mesonephric ducts (also called wolffian ducts), which is the male duct system, and the paramesonephric, ducts (also called mullerian ducts) that form the female reproductive ducts. So the newly developing testes produce testosterone to promote mesonephric duct development or the male genitalia, and suppress the mesonephric ducts (also called wolffian ducts), which is the male duct system, and the paramesonephric, ducts (also called mullerian ducts) that form the female reproductive ducts. So the newly developing testes produce testosterone to promote mesonephric duct development or the male reproductive system, and suppress paramesonephric development, which feminizes the reproduction systems development, which feminizes the reproduction system.
-Each embryonic testis must descend from its posterior abdomen location through the inguinal canals located in the anterior abdomen. The testes begin their migration to the scrotum at about the twenty-eighth week lasting 2 or 3 days. About week 32 the testes are fully descended into the scrotum in 97 percent of males and shortly after birth for the remainder three percent. For the 3 percent of full-term males who may have an undescended testis the testis should complete migration in the first year post gestation. Cryptorchidism, the medical term for undescended testis, is a pathological condition in newborns and requires medical or surgical attention when it persists.
-The risk of malignant testicular tumor with cryptorchidism is 10 to 40 times normal descended testes. The higher in the abdomen the testis the greater the risk, and if both are undescended versus only one undescended.
-The epididymides are paired organs described as a “comma-shaped” structure along the superior and posterolateral surface of each testicle. Named parts of the epididymis are the head, body, and tail.
-The process involves reduction division of the human gene complement by a process called meiosis. Meiosis is a very efficient process producing thousands of sperm each second in healthy males. More than 100 million sperm are produced each day in normal fertile testes. From the beginning of meiosis to full maturation is about 2 month.
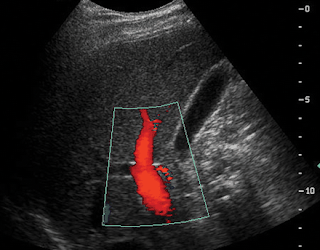
-Knowing the normal texture of the testes is important to the sonographer. The normal testis appears homogenous on ultrasound with an echo texture similar to the thyroid gland. The normal testis appears encapsulated owing this presentation to a hypoechoic ring, which is the tunica vaginalis.
-Structures to be demonstrated are: the mediastinum of the testis, rete testis, head of the epididymis (body and tail with hydrocele), and testis. The mediastinum of the testis is often seen as an echogenic linear band with longitudinal imaging of the testicle. The rete testis is visualized as a hypoechoic or septated cystic area near the head of the epididymis.
-The epididymis is distinguished from the testicle by it being isoechoic or slightly more echogenic than the testis. The echotexture looks coarse compared to the adjacent testis.
-Doppler imaging with ultrasound is a technique for demonstrating the blood flow to the testis and epididymis. Maximum effectiveness of Doppler imaging of the testis for ischemia is achieved when images are acquired at early suspicion of ischemia. The effectiveness diminishes as reactive scrotal inflammation occurs in conditions like torsion.
-Color Doppler (CD) is an imaging technique that allows the sonographer to demonstrate blood flow images in color placed over real time B-mode images. Red is allocated for flow towards the transducer and blue is allocated for blood flow away from the transducer. To measure what type of vessel is being demonstrated pulsed wave Doppler (PWD) can be used.
-Pulsed wave Doppler technique can be used to determine whether a vessel is arterial or venous by observing the waveform pattern shows the signal as being either above or below baseline. High resistance shows high systolic peak and low diastolic flow. Low resistance shows a double or biphasic systolic flow and high diastolic flow.
-It is important for the sonographer to know the appearance of a normal Doppler waveform for blood flow to the testicles. The normal arterial Doppler waveform will have a low-impedance for the testicular artery, and a large amount of end diastolic flow within the artery. The diferential and cremasteric arteries are found within the spermatic cord, and should show a high resistance waveform and an absence of diastolic flow.
-Blood flow in the testicular artery is low resistance. Blood flow in the cremasteric artery is high resistance, and flow in the diferential artery is high resistance.
-A hydrocele is a collection of fluid in the tunica vaginalis. It is a common cause of painless scrotal enlargement. A pyelocele is pus in the tunica vaginalis. Blood and pus within the scrotal sac is frequently accompanied with pain. A collection of lymphatic fluid in the tunica vaginalis is a chylocele.
-A varicocele is caused when veins within the spermatic cord becomes excessively dilated resulting in a cystic-like varix. The primary cause is incompetent valves of the pampiniform plexus, but can also result from a blockage of the testicular veins or renal veins. Patients often describe their situation as having two testicles on one side. This condition most commonly affects the left testicle approximately 80% of the time.
-Varicocele can be demonstrated with color Doppler; however, the sonographer must have the patient perform the “Valsalva technique,” which involves suspended inspiration is used to temporary increase venous pressure.
-Epididymitis is the inflammation of the epididymis and is the most common cause of acute scrotal pain. Acute epididymitis appears hypoechoic on ultrasound, and Doppler shows increased blood flow. Chronic epididymitis presents differently, it shows enlargement and areas that are affected will be hyperechoic.
-Orchitis is inflammation of the testes usually related to and secondary to epididymitis. It commonly occurs because of an infection in the urinary tract (cystitis, urethritis, and genitoprostatitis) that seeds to the epididymis and testis through the lymphatics or ductus deferens. It also can result from mumps, trauma or autoimmune reaction.
-Testicular microlithiasis (TM) is seen on approximately 0.6% of testicular sonograms. Microlithiasis describes calcifications found inside the seminiferous tubules or testicles and is a very uncommon condition. Ultrasound shows several, small hyperechoic foci scattered through out the testicle. These tiny punctate echogenic foci may be easy to recognize because they do not typically shadow. Microlithiasis has been associated with testicular cancer so those patients with this condition should be watched closely.
-Testicular tumors characteristically spread through lymphatic channels first affecting retroperitoneal and para-aortic nodes. From retroperitoneal nodes it spreads to the mediastinal and subclavicular nodes. When hematogenous spread occurs the lungs are the primary organs affected. Secondary hematogenous spread may involve the liver, brain or bone.
-The main difference between seminoma and NSGCT is that seminomas tend to stay localized to the testis. Because the stay localized for a long time over 70% are discovered while still within the testis. Seminomas are highly radiosensitive accounting for a high cure rate of 85-95%.
-Certain serum tumor markers are elevated when there is a testicular tumor. This is because certain germ cell tumors secrete hormones and specific enzymes detectable by laboratory tests. Some of these bio-molecular markers include: human chorionic gonadotropin (HCG), alpha fetal protein (AFP), lactic dehydrogenase, placental lactogen, and placental alkaline phosphatase. The two most reliable ones are AFP and Beta-HGC.
-Choriocarcinoma is another type of neoplasm that occurs in males 20-30 years of age. It is the least common type of neoplasm accounting for 1-3% of germ cell tumors. However, it is the most malignant of testicular cancers killing almost all its victims within five years of diagnosis.
-Teratomas comprise about 10-15% of germ cell tumors. They are composed of all three germ layers, the mesoderm, ectoderm, and endoderm tissues. About a third of teratomas of the testicle will metastasize through lymphatic spread within 5 years of discovery.
-About 40% of testicular tumors are mixed; the most common mix is teratoma and embryonal carcinoma. Alpha-Fetoprotein is raised when embryonal carcinoma is present, and beta-HCG is found in over 50% of nonseminomatous tumors and some pure seminomas.
http://www.ceessentials.net/article42.html
Friday, July 23, 2010
Wednesday, July 21, 2010
 Aliasing of the spectral waveform. Duplex US image shows aliasing of the spectral waveform with wraparound of the highest flow velocities into the negative part of the graph. Note that the color Doppler flow US image shows normal antegrade portal venous flow with no aliasing. To eliminate or reduce this artifact, spectral Doppler US must be active before different parameters can be modified.
Aliasing of the spectral waveform. Duplex US image shows aliasing of the spectral waveform with wraparound of the highest flow velocities into the negative part of the graph. Note that the color Doppler flow US image shows normal antegrade portal venous flow with no aliasing. To eliminate or reduce this artifact, spectral Doppler US must be active before different parameters can be modified.http://radiographics.rsna.org/content/24/3/657.figures-only

 Changing the color baseline to avoid aliasing. (a) On a color Doppler flow US image, flow within the portal vein appears green, the color equivalent of aliasing on the selected color bar. The color baseline (arrow) is positioned too high on the color bar. Although the US image helps confirm the presence of flow, the baseline should be lowered to obtain meaningful directional flow data. (b) On a color Doppler flow US image obtained after the baseline was lowered (arrow), accurate directional flow data have been obtained from the main portal vein: Appropriate antegrade portal venous flow toward the transducer appears red.
Changing the color baseline to avoid aliasing. (a) On a color Doppler flow US image, flow within the portal vein appears green, the color equivalent of aliasing on the selected color bar. The color baseline (arrow) is positioned too high on the color bar. Although the US image helps confirm the presence of flow, the baseline should be lowered to obtain meaningful directional flow data. (b) On a color Doppler flow US image obtained after the baseline was lowered (arrow), accurate directional flow data have been obtained from the main portal vein: Appropriate antegrade portal venous flow toward the transducer appears red. 
 Changing the spectral baseline to avoid aliasing. (a) Duplex US image demonstrates aliasing of the spectral waveform, which results in the production of inaccurate waveform data and an inability to obtain accurate quantitative flow data. (b) On a duplex US image obtained after the spectral baseline was lowered, the spectral waveform falls within the range of velocities being evaluated, so that accurate quantitative data can be obtained. Note that changing the baseline does not change the velocity scale (PRF = 1,515 Hz), making this adjustment a logical initial change when reducing aliasing.
Changing the spectral baseline to avoid aliasing. (a) Duplex US image demonstrates aliasing of the spectral waveform, which results in the production of inaccurate waveform data and an inability to obtain accurate quantitative flow data. (b) On a duplex US image obtained after the spectral baseline was lowered, the spectral waveform falls within the range of velocities being evaluated, so that accurate quantitative data can be obtained. Note that changing the baseline does not change the velocity scale (PRF = 1,515 Hz), making this adjustment a logical initial change when reducing aliasing. 
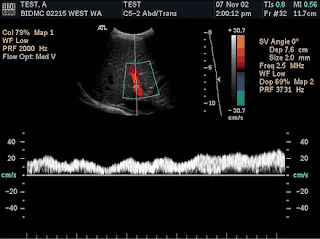 Adjusting the spectral scale. (a) Color duplex US image demonstrates that when the spectral scale (or the sampling rate) is set too high (in this example, PRF = 14,286 Hz), flow is more difficult to appreciate and characterize on the scale. (b) On a color duplex US image obtained after the scale was reduced (PRF = 3,731 Hz), the range of depicted velocities is reduced and the appearance of the spectral waveform is improved, providing more visible quantitative and qualitative data. Note that the color Doppler flow US image, color bar, and color scale all remain unchanged because the spectral component is active.
Adjusting the spectral scale. (a) Color duplex US image demonstrates that when the spectral scale (or the sampling rate) is set too high (in this example, PRF = 14,286 Hz), flow is more difficult to appreciate and characterize on the scale. (b) On a color duplex US image obtained after the scale was reduced (PRF = 3,731 Hz), the range of depicted velocities is reduced and the appearance of the spectral waveform is improved, providing more visible quantitative and qualitative data. Note that the color Doppler flow US image, color bar, and color scale all remain unchanged because the spectral component is active. 

 Adjusting the color velocity scale. (a) Color Doppler flow US image obtained with the color velocity scale set too high (69.2 cm/sec) demonstrates apparent absence of flow in the portal vein. (b) Color Doppler flow US image obtained after the scale was reduced to 30.7 cm/sec demonstrates normal flow in a widely patent portal vein. (c) Color Doppler flow US image obtained after the scale was set even lower (2.3 cm/sec) shows aliasing of color flow in all branches of the portal vein, which results in meaningless data concerning flow direction. Thus, the color velocity scale should be increased to increase the sampling rate.
Adjusting the color velocity scale. (a) Color Doppler flow US image obtained with the color velocity scale set too high (69.2 cm/sec) demonstrates apparent absence of flow in the portal vein. (b) Color Doppler flow US image obtained after the scale was reduced to 30.7 cm/sec demonstrates normal flow in a widely patent portal vein. (c) Color Doppler flow US image obtained after the scale was set even lower (2.3 cm/sec) shows aliasing of color flow in all branches of the portal vein, which results in meaningless data concerning flow direction. Thus, the color velocity scale should be increased to increase the sampling rate. 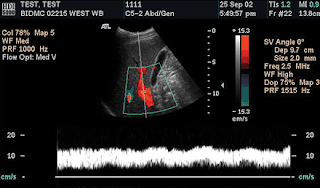
 Changing the wall filter. (a) Color duplex US image obtained with a high wall filter setting shows loss of the low-velocity-flow component of the spectral waveform immediately above the baseline. Higher-velocity flow is well depicted, and accurate flow quantification can still occur. In the evaluation of the liver vasculature, this is likely to become relevant only when flow velocity is very low and falls within the range of velocities that are filtered out. (b) Color duplex US image demonstrates how the spectral waveform progressively fills in toward the baseline as the wall filter is sequentially reduced from high (left arrow) to medium (middle arrow) to low (right arrow).
Changing the wall filter. (a) Color duplex US image obtained with a high wall filter setting shows loss of the low-velocity-flow component of the spectral waveform immediately above the baseline. Higher-velocity flow is well depicted, and accurate flow quantification can still occur. In the evaluation of the liver vasculature, this is likely to become relevant only when flow velocity is very low and falls within the range of velocities that are filtered out. (b) Color duplex US image demonstrates how the spectral waveform progressively fills in toward the baseline as the wall filter is sequentially reduced from high (left arrow) to medium (middle arrow) to low (right arrow). 
 Changing the color Doppler wall filter. (a) Color Doppler flow US image obtained with the highest possible wall filter setting shows how color signal arising from low-velocity flow may be filtered out. (b) Color Doppler flow US image obtained with a low filter setting demonstrates filling in of flow in the hepatic veins (blue), which indicates minimal filtering of color signal. The change in the filter setting appears as a change in the width of the horizontal black line in the center of the color bar.
Changing the color Doppler wall filter. (a) Color Doppler flow US image obtained with the highest possible wall filter setting shows how color signal arising from low-velocity flow may be filtered out. (b) Color Doppler flow US image obtained with a low filter setting demonstrates filling in of flow in the hepatic veins (blue), which indicates minimal filtering of color signal. The change in the filter setting appears as a change in the width of the horizontal black line in the center of the color bar.
 Inversion of color flow. (a) On a color Doppler flow US image obtained with color Doppler flow US as the active scanning mode and inversion of the color bar, portal venous flow appears blue, which falsely suggests reversal of flow (ie, away from the transducer). (b) On a color Doppler flow US image obtained with reversal of this inversion, appropriate directional flow is noted.
Inversion of color flow. (a) On a color Doppler flow US image obtained with color Doppler flow US as the active scanning mode and inversion of the color bar, portal venous flow appears blue, which falsely suggests reversal of flow (ie, away from the transducer). (b) On a color Doppler flow US image obtained with reversal of this inversion, appropriate directional flow is noted.
 Inversion of spectral and color flow falsely suggesting reversal of portal venous flow. (a) On a color duplex US image obtained with spectral Doppler US as the active scanning mode, the spectral waveform is below the baseline, with appropriate color flow. (b) Color duplex US image obtained after the inversion button was reversed demonstrates appropriate directional flow, with the spectral waveform now appearing above the baseline. Note that the color bar does not change when the Doppler spectrum is inverted.
Inversion of spectral and color flow falsely suggesting reversal of portal venous flow. (a) On a color duplex US image obtained with spectral Doppler US as the active scanning mode, the spectral waveform is below the baseline, with appropriate color flow. (b) Color duplex US image obtained after the inversion button was reversed demonstrates appropriate directional flow, with the spectral waveform now appearing above the baseline. Note that the color bar does not change when the Doppler spectrum is inverted. 
 Angle correction. (a) Color duplex US image obtained with no angle correction shows how no meaningful velocity data can be obtained from the portal venous waveform because the computer automatically assigns an angle of 0° (cos 0° = 1). Without angle correction, the measured flow velocity is 18.0 cm/sec. (b) Color duplex US image obtained with correct definition of the angle between the transducer and the direction of portal venous flow demonstrates a flow velocity of 29.3 cm/sec.
Angle correction. (a) Color duplex US image obtained with no angle correction shows how no meaningful velocity data can be obtained from the portal venous waveform because the computer automatically assigns an angle of 0° (cos 0° = 1). Without angle correction, the measured flow velocity is 18.0 cm/sec. (b) Color duplex US image obtained with correct definition of the angle between the transducer and the direction of portal venous flow demonstrates a flow velocity of 29.3 cm/sec. 
 Angle correction. (a) Color duplex US image obtained with a 30° corrected angle, which is too low, demonstrates a flow velocity of 21.3 cm/sec in the portal vein. This figure represents an underestimation of the true flow velocity. (b) Color duplex US image obtained with a 70° corrected angle, which is too high, demonstrates a flow velocity of 52.8 cm/sec in the portal vein, which represents an overestimation of flow velocity. Note that the measured flow velocity increases as the corrected angle increases.
Angle correction. (a) Color duplex US image obtained with a 30° corrected angle, which is too low, demonstrates a flow velocity of 21.3 cm/sec in the portal vein. This figure represents an underestimation of the true flow velocity. (b) Color duplex US image obtained with a 70° corrected angle, which is too high, demonstrates a flow velocity of 52.8 cm/sec in the portal vein, which represents an overestimation of flow velocity. Note that the measured flow velocity increases as the corrected angle increases. 

 Angle of insonation. (a, b) Color duplex US images of the anterior branch of the right portal vein obtained with the transducer positioned in an intercostal (a) and subcostal (b) location depict flow as moving toward and away from the transducer, respectively. (c) On a color duplex US image obtained with the transducer positioned perpendicular to flow (arrow), no color is assigned, yielding a false finding of absent flow. The angle of insonation of the vein depends entirely on the position of the transducer.
Angle of insonation. (a, b) Color duplex US images of the anterior branch of the right portal vein obtained with the transducer positioned in an intercostal (a) and subcostal (b) location depict flow as moving toward and away from the transducer, respectively. (c) On a color duplex US image obtained with the transducer positioned perpendicular to flow (arrow), no color is assigned, yielding a false finding of absent flow. The angle of insonation of the vein depends entirely on the position of the transducer. 


 Optimization of gain settings. (a) Duplex US image obtained with spectral Doppler US as the active scanning mode and too low a gain setting (0%) falsely suggests absent flow. (b-d) Duplex US images obtained with a gain setting of 38% (b), 77% (c), and 100% (d) demonstrate gradual artificial filling in of the spectral waveform, yielding a false finding of increased flow with little meaningful quantitative flow data. The gain settings function independently of other parameters; therefore, changing the gain setting will not alter any other parameter. Changing the color gain does not alter the spectral gain (and vice versa), and the PRF remains unchanged. Whether the color or spectral component is active, the gain setting should be adjusted to outline the contour of the depicted waveform or color flow depiction.
Optimization of gain settings. (a) Duplex US image obtained with spectral Doppler US as the active scanning mode and too low a gain setting (0%) falsely suggests absent flow. (b-d) Duplex US images obtained with a gain setting of 38% (b), 77% (c), and 100% (d) demonstrate gradual artificial filling in of the spectral waveform, yielding a false finding of increased flow with little meaningful quantitative flow data. The gain settings function independently of other parameters; therefore, changing the gain setting will not alter any other parameter. Changing the color gain does not alter the spectral gain (and vice versa), and the PRF remains unchanged. Whether the color or spectral component is active, the gain setting should be adjusted to outline the contour of the depicted waveform or color flow depiction.  Optimizing gate size and position. Color duplex US image obtained with a wide gate placed in a suboptimal location shows sampling of flow in both the portal (above the baseline) and hepatic (below the baseline) veins. Too large a gate size may result in sampling from too large an anatomic region. By reducing the gate size and improving the position for sampling, a normal spectral waveform is obtained.
Optimizing gate size and position. Color duplex US image obtained with a wide gate placed in a suboptimal location shows sampling of flow in both the portal (above the baseline) and hepatic (below the baseline) veins. Too large a gate size may result in sampling from too large an anatomic region. By reducing the gate size and improving the position for sampling, a normal spectral waveform is obtained.http://radiographics.rsna.org/content/24/3/657.figures-only
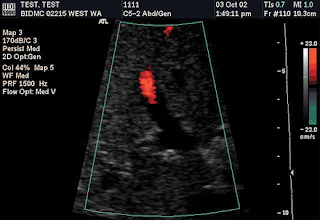

 Adjustment of color gain with color flow US as the active scanning mode. (a, b) Color Doppler flow US images obtained with a gain setting of 44% (a) and 100% (b) show underadjustment and overadjustment, respectively. (c) Color Doppler flow US image obtained with an optimal gain setting of 65% demonstrates normal-appearing wall-to-wall flow in the main portal vein. Note that, although the color gain changes, no change occurs in the color velocity scale (23 cm/sec) or sampling rate (PRF = 1,500 Hz).
Adjustment of color gain with color flow US as the active scanning mode. (a, b) Color Doppler flow US images obtained with a gain setting of 44% (a) and 100% (b) show underadjustment and overadjustment, respectively. (c) Color Doppler flow US image obtained with an optimal gain setting of 65% demonstrates normal-appearing wall-to-wall flow in the main portal vein. Note that, although the color gain changes, no change occurs in the color velocity scale (23 cm/sec) or sampling rate (PRF = 1,500 Hz).  Color baseline. The position of the baseline on the color bar is indicated by a horizontal black line (yellow circles). When the baseline is adjusted, the relative position of this horizontal black line changes. Note that when the position of the baseline is changed, the color velocity range that is displayed on the color bar also changes (in this example, from 15.3 to 46.1 cm/sec above or below the baseline). The range of depicted velocities remains constant, but different flow velocities will be emphasized depending on their relative position on the color bar.
Color baseline. The position of the baseline on the color bar is indicated by a horizontal black line (yellow circles). When the baseline is adjusted, the relative position of this horizontal black line changes. Note that when the position of the baseline is changed, the color velocity range that is displayed on the color bar also changes (in this example, from 15.3 to 46.1 cm/sec above or below the baseline). The range of depicted velocities remains constant, but different flow velocities will be emphasized depending on their relative position on the color bar.http://radiographics.rsna.org/content/24/3/657.figures-only
Tuesday, July 20, 2010
 Reversal of left portal venous flow in a patient with a TIPS. Color Doppler flow US image shows flow toward the transducer (red) in the left hepatic artery (HA) and reversed flow (blue) in the left portal vein (LPV). These findings are expected when a functioning TIPS bridges the right portal and hepatic veins.
Reversal of left portal venous flow in a patient with a TIPS. Color Doppler flow US image shows flow toward the transducer (red) in the left hepatic artery (HA) and reversed flow (blue) in the left portal vein (LPV). These findings are expected when a functioning TIPS bridges the right portal and hepatic veins.http://www.google.com.eg/imgres?imgurl=http://radiographics.rsna.org/content/24/3/657/F43.small.gif&imgrefurl=http://radiographics.rsna.org/content/24/3/657.full&usg=__F7-OEFZQVV-SS9oqhkkqgRTZCIk=&h=135&w=200&sz=24&hl=ar&start=4&tbnid=hbLUfp8WZCGr-M:&tbnh=70&tbnw=104&prev=/images%3Fq%3Dvenous%2Bspectral%2Bwave%2Bform%26hl%3Dar%26sa%3DG%26gbv%3D2%26tbs%3Disch:1&itbs=1
 Normal hepatic venous waveform. On a color duplex US image, the spectral waveform for a normal hepatic vein shows triphasic flow above and below the baseline. The waveform shows periodicity and is triphasic due to transmitted cardiac activity, similar to the waveform for the jugular vein. The component above the baseline corresponds to atrial systole; the components below the baseline correspond to ventricular systole and the filling phase during atrial diastole.
Normal hepatic venous waveform. On a color duplex US image, the spectral waveform for a normal hepatic vein shows triphasic flow above and below the baseline. The waveform shows periodicity and is triphasic due to transmitted cardiac activity, similar to the waveform for the jugular vein. The component above the baseline corresponds to atrial systole; the components below the baseline correspond to ventricular systole and the filling phase during atrial diastole.http://www.google.com.eg/imgres?imgurl=http://radiographics.rsna.org/content/24/3/657/F43.small.gif&imgrefurl=http://radiographics.rsna.org/content/24/3/657.full&usg=__F7-OEFZQVV-SS9oqhkkqgRTZCIk=&h=135&w=200&sz=24&hl=ar&start=4&tbnid=hbLUfp8WZCGr-M:&tbnh=70&tbnw=104&prev=/images%3Fq%3Dvenous%2Bspectral%2Bwave%2Bform%26hl%3Dar%26sa%3DG%26gbv%3D2%26tbs%3Disch:1&itbs=1
normal venous characteristics
The normal venous wave form characterised by the following:
1-Spontaneous flow:
Continuous flow of phasic wave form.
2-Phasic flow:
The amplitude of the wave formed controlled by respiration
during inspiration,the intra-abdominal pressure is increased by descending of diaphragm resulting in cessation of blood flow inside the vein.
while in expiration,the intra abdominal pressure is decreased resulting in accentuation of the peak of the wave form.
If the examiner for example is situating the probe in the groin lesion and he found that the doppler wave form of the common femoral vein is monophasic,this will indicate a lesion situated between the site of examination and pelvic veins.
3-Distal augmentation:
squeesing or compression distal to the site of examination result in augmentation of doppler venous wave form.this indicate patency of the segment of the vein between the distal site of compression and the site where the probe is situated.
4-Non pulsatility:
-Normally veins are non pulsatile.
-It could be pulsatile in the following situations
a) cardiac diseases.
b) severe brady-cardia.
c) over-transfusion.
5-Compressability (the most important sign )
-Normal veins are thin walled and easly compressible.
-This is one of the most criteria for venous examination.
-When an external compression is applied to a vein using ultrasoud probe
a) if the vein is healthy
walls of the vein will be collapsed completely resulting in opposed walls.
b) partially occluded vein will be partially collapsed while
c) Total occluded vein will not collapse at all.
Demonstration of non collapsed vein indicate presence of venous thrombi.
6-Competency of valves.
-Can be demonstrated by one of the following
a) application of valsalva maneuver. or
b) compression maneuver proximal to the site of probe application.
-After application of one of these maneuvers,venous flow should be stopped untill this maneuver is discontinued.
-There should be no reversal of flow.
In case of competent vein:
-you will found only one sound.
-spectral doppler wave form will be in one direction.
-color doppler image will show one color.
In case of incompetent vein:
-there is two sounds ,forward and reverse as blood flow in two directions.
-spectral wave form will shows two direction of flow
-color doppler image will shows two color.
http://www.radiologyspirit.blogspot.com/
1-Spontaneous flow:
Continuous flow of phasic wave form.
2-Phasic flow:
The amplitude of the wave formed controlled by respiration
during inspiration,the intra-abdominal pressure is increased by descending of diaphragm resulting in cessation of blood flow inside the vein.
while in expiration,the intra abdominal pressure is decreased resulting in accentuation of the peak of the wave form.
If the examiner for example is situating the probe in the groin lesion and he found that the doppler wave form of the common femoral vein is monophasic,this will indicate a lesion situated between the site of examination and pelvic veins.
3-Distal augmentation:
squeesing or compression distal to the site of examination result in augmentation of doppler venous wave form.this indicate patency of the segment of the vein between the distal site of compression and the site where the probe is situated.
4-Non pulsatility:
-Normally veins are non pulsatile.
-It could be pulsatile in the following situations
a) cardiac diseases.
b) severe brady-cardia.
c) over-transfusion.
5-Compressability (the most important sign )
-Normal veins are thin walled and easly compressible.
-This is one of the most criteria for venous examination.
-When an external compression is applied to a vein using ultrasoud probe
a) if the vein is healthy
walls of the vein will be collapsed completely resulting in opposed walls.
b) partially occluded vein will be partially collapsed while
c) Total occluded vein will not collapse at all.
Demonstration of non collapsed vein indicate presence of venous thrombi.
6-Competency of valves.
-Can be demonstrated by one of the following
a) application of valsalva maneuver. or
b) compression maneuver proximal to the site of probe application.
-After application of one of these maneuvers,venous flow should be stopped untill this maneuver is discontinued.
-There should be no reversal of flow.
In case of competent vein:
-you will found only one sound.
-spectral doppler wave form will be in one direction.
-color doppler image will show one color.
In case of incompetent vein:
-there is two sounds ,forward and reverse as blood flow in two directions.
-spectral wave form will shows two direction of flow
-color doppler image will shows two color.
http://www.radiologyspirit.blogspot.com/
Monday, July 19, 2010
1-Begin by simply looking at the area in question, which is on either side of the crease separating the leg from the groin region. Make note of any discrete swellings, which might represent adenopathy or a femoral hernia.
2-Palpate the area, feeling carefully for the femoral pulses as well as for inguinal/femoral adenopathy (nodes which surround the femoral artery and vein.... up to one cm in size are considered non-pathologic). If you feel any lymph nodes, note if they are firm or soft, fixed in position or freely mobile (fixed, firm nodes are more worrisome for pathologic states).
3-The femoral pulse should be easily identifiable, located along the crease midway between the pubic bone and the anterior iliac crest. Use the tips of your 2nd, 3rd and 4th fingers. If there is a lot of subcutaneous fat, you will need to push firmly.
4-A femoral hernia, if present, is located on the anterior thigh, medial to the femoral artery. As it can be transient (i.e. the patient reports its presence yet you find nothing on examination), investigation should include observation as well as palpation while the patient performs a valsalva maneuver, which may make a hernia more prominent.

2-Palpate the area, feeling carefully for the femoral pulses as well as for inguinal/femoral adenopathy (nodes which surround the femoral artery and vein.... up to one cm in size are considered non-pathologic). If you feel any lymph nodes, note if they are firm or soft, fixed in position or freely mobile (fixed, firm nodes are more worrisome for pathologic states).
3-The femoral pulse should be easily identifiable, located along the crease midway between the pubic bone and the anterior iliac crest. Use the tips of your 2nd, 3rd and 4th fingers. If there is a lot of subcutaneous fat, you will need to push firmly.
4-A femoral hernia, if present, is located on the anterior thigh, medial to the femoral artery. As it can be transient (i.e. the patient reports its presence yet you find nothing on examination), investigation should include observation as well as palpation while the patient performs a valsalva maneuver, which may make a hernia more prominent.

Location of Dorsalis Pedis Artery
The pictures below demonstrate the location of the dorsalis pedis artery in relation to surrounding structures (surface anatomy on above, gross anatomy on below).
Subscribe to:
Posts (Atom)