
Saturday, July 17, 2010

Sensitivity and Specificity of Colour Duplex Ultrasonography

 Some perforating vein groups important for surgical treatment of venous insufficiency, seen from behind. (Dodd's group are venae comm. fem. med. intermedia, Boyd's are the vv. comm. cruris intermed., and Cockett's group empty into a vein of the vv. tib. post.). The levels of two dorsal perforators are also indicated, the upper communicating with a gastrocnemius vein.
Some perforating vein groups important for surgical treatment of venous insufficiency, seen from behind. (Dodd's group are venae comm. fem. med. intermedia, Boyd's are the vv. comm. cruris intermed., and Cockett's group empty into a vein of the vv. tib. post.). The levels of two dorsal perforators are also indicated, the upper communicating with a gastrocnemius vein. Schematic drawing of the left pelvic and lower extremity veins. The deep leg veins from the level of the popliteal vein are paired (not shown). The gastrocnemius veins are paired and duplicated; there are several soleal veins.
Schematic drawing of the left pelvic and lower extremity veins. The deep leg veins from the level of the popliteal vein are paired (not shown). The gastrocnemius veins are paired and duplicated; there are several soleal veins.www.medcyclopaedia.com/.../nic_k201_000.jpg
surgical anatomy of saphenous veins
 Figure 1. Superficial veins: LSV; long saphenous vein, SSV; short saphenous vein, SFJ; saphenofemoral junction, PAV; posterior arch vein, SPJ; saphenopopliteal junction. (Picture drawn by Pentti Rautio)
Figure 1. Superficial veins: LSV; long saphenous vein, SSV; short saphenous vein, SFJ; saphenofemoral junction, PAV; posterior arch vein, SPJ; saphenopopliteal junction. (Picture drawn by Pentti Rautio)occluded great saphenous vein
 Sonogram and cartoon showing an open saphenofemoral junction with a short patent segment above an otherwise occluded great saphenous vein (GSV). The superficial external pudendal (SEP) vein is patent with normal, prograde flow through the SFJ. CFV, Common femoral vein.
Sonogram and cartoon showing an open saphenofemoral junction with a short patent segment above an otherwise occluded great saphenous vein (GSV). The superficial external pudendal (SEP) vein is patent with normal, prograde flow through the SFJ. CFV, Common femoral vein.http://www.google.com.eg/imgres?imgurl=http://www.veinsurg.com/gal/biblio/chir/chir_7/fig1.jpg&imgrefurl=http://www.veinsurg.com/fr/biblio/chirurgie/chirurgie_7.php&usg=__kayhyYvmS0h6O5tMoixfyZxDgCg=&h=561&w=808&sz=119&hl=ar&start=20&tbnid=wQdy4jSjGeefoM:&tbnh=99&tbnw=143&prev=/images%3Fq%3Dhunterian%2Bperforator%26hl%3Dar%26sa%3DG%26gbv%3D2%26tbs%3Disch:1&itbs=1
great saphenous vein reflux
 great saphenous vein reflux
great saphenous vein refluxa, Pretreatment duplex image showing midthigh great saphenous vein reflux. Note diameter markers. b, Same vein level at 6 mo, showing diameter reduction, vein wall thickening, and a narrow, irregular, echolucent lumen without flow. c, Same level at 2 years, now seen as a featureless hyperechogenic stripe with further diameter shrinkage and no discernible lumen.
http://www.google.com.eg/imgres?imgurl=http://www.veinsurg.com/gal/biblio/chir/chir_7/fig1.jpg&imgrefurl=http://www.veinsurg.com/fr/biblio/chirurgie/chirurgie_7.php&usg=__kayhyYvmS0h6O5tMoixfyZxDgCg=&h=561&w=808&sz=119&hl=ar&start=20&tbnid=wQdy4jSjGeefoM:&tbnh=99&tbnw=143&prev=/images%3Fq%3Dhunterian%2Bperforator%26hl%3Dar%26sa%3DG%26gbv%3D2%26tbs%3Disch:1&itbs=1
 Small Saphenous Vein (SSV)
Small Saphenous Vein (SSV)Courses from the lateral ankle up the posterior calf
Terminates in the popliteal fossa at the saphenopopliteal junction (SPJ)
Confluence with the popliteal vein (PV) is variable
Proximal portion lies between superficial & deep fascial layers
http://www.google.com.eg/imgres?imgurl=http://www.icvein.com/images/saphenous-vein.jpg&imgrefurl=http://www.icvein.com/venous-disease-varicose-vein-treatment-iowa-city-ia.htm&usg=__Ne7smwjRmou-16vDYSuoafZgyNI=&h=385&w=221&sz=19&hl=ar&start=3&tbnid=jKodCR2axztKAM:&tbnh=123&tbnw=71&prev=/images%3Fq%3Dlesser%2Bsaphenous%2Bvein%2Bperforators%26hl%3Dar%26sa%3DG%26gbv%3D2%26tbs%3Disch:1&itbs=1
 Connect superficial to deep veins
Connect superficial to deep veinsLocations
Proximal thigh - Hunterian
Distal thigh - Dodd’s
Knee - Boyd’s
Ankle/Calf - Cockett’s
Incompetent perforators often source of venous stasis ulcers at medial ankle
http://www.google.com.eg/imgres?imgurl=http://www.icvein.com/images/saphenous-vein.jpg&imgrefurl=http://www.icvein.com/venous-disease-varicose-vein-treatment-iowa-city-ia.htm&usg=__Ne7smwjRmou-16vDYSuoafZgyNI=&h=385&w=221&sz=19&hl=ar&start=3&tbnid=jKodCR2axztKAM:&tbnh=123&tbnw=71&prev=/images%3Fq%3Dlesser%2Bsaphenous%2Bvein%2Bperforators%26hl%3Dar%26sa%3DG%26gbv%3D2%26tbs%3Disch:1&itbs=1


1-Anatomy
The superficial veins lie in the subcutaneous fatty layer of the body just beneath the skin and superficial to the deep fascia enveloping the body musculature. The principal veins in the legs are the great and lesser saphenous veins and their tributaries; in the arms they are the basilic and cephalic veins and their tributaries. The deep veins accompany arteries and bear the same name as the arteries they parallel. It is common in the extremities for there to be two or more veins accompanying a small- to medium-sized artery. The perforating veins penetrate the deep fascia and connect the superficial veins to the deep veins. Those along the inner (medial) side of the lower leg play a major role in the pathogenesis of the "postphlebitic leg". The intra-muscular sinusoidal veins are large, very thin walled, valveless veins within skeletal muscle. They connect directly with the deep veins.
Pathophysiology
 Figure 1 - Long and short saphenous system
Figure 1 - Long and short saphenous systemThe power to drive the blood back up the leg is provided by the calf muscle, which on walking contracts and relaxes in a regular movement. The contraction of the calf muscle forces the blood upward out of a segment of vein; backflow is prevented by the valve [7]. Relaxation of the calf muscle allows the now empty segment of deep vein to refill with blood from the superficial veins and thus the cycle is repeated.
When valves become incompetent the cycle of unidirectional blood flow is interrupted and backflow of blood occurs (Figure 2). This is most significant when the backflow occurs between the deep and superficial veins, as the increased pressure in the superficial veins will cause further valve incompetence. This is because the valve cusps no longer meet as a result of the stretching of the veins. The overall effect of this increased superficial hydrostatic pressure is the formation of tortuous varicose veins [9].
 Figure 2 - Venous system (normal and damaged)
Figure 2 - Venous system (normal and damaged)www.worldwidewounds.com/2002/september/Johnso...
Friday, July 16, 2010
 Measuring the ankle-brachial index (ABI). The ABI is 95% sensitive and 99% specific for angiographically measured lower extremity arterial stenosis of 50% or greater. The ABI is calculated as the ratio of Doppler recorded systolic pressures in the lower and upper extremities. DP=dorsalis pedis; PT=posterior tibial. Adapted with permission of the Massachusetts Medical Society from N Engl J Med. 2001;344:1608-1621.[47]
Measuring the ankle-brachial index (ABI). The ABI is 95% sensitive and 99% specific for angiographically measured lower extremity arterial stenosis of 50% or greater. The ABI is calculated as the ratio of Doppler recorded systolic pressures in the lower and upper extremities. DP=dorsalis pedis; PT=posterior tibial. Adapted with permission of the Massachusetts Medical Society from N Engl J Med. 2001;344:1608-1621.[47]Assessing the lower-limb arteries
 Figure 7: The main arteries of the lower limb.
Figure 7: The main arteries of the lower limb.Patients are normally referred with claudication (pain on walking, particularly uphill) or with ulcers around the ankle and on clinical examination the ankle pulses are likely to be weak or absent. The main options for treatment are either (a) to encourage the patient to exercise and develop collateral vessels to take blood around the site of disease, (b) to perform angioplasty which involves passing a balloon catheter down the artery and inflating it at the sites of narrowing or blockage to dilate the vessel, or (c) to surgically insert an graft to bypass the diseased portion of the vessel [5] . The clinical information required is therefore the site and severity of any narrowing of the vessel and the site and length of any blockages of the main vessels. In the presence of multiple stenoses, the study can indicate which are causing the more critical restrictions to blood flow. The referring clinician can use this information to decide whether intervention is appropriate, and, if so, whether to proceed to angioplasty or to insert a graft [6] .
The examination starts with the patient lying supine with the head slightly raised. Coupling gel is put on the thigh from groin to knee over the path of the artery. The probe is placed on the skin in the groin and the common femoral artery identified using the colour Doppler display. The scanner is switched to duplex mode, and the blood velocity waveform in the common femoral artery is obtained. The waveform is usually biphasic or triphasic. The peak systolic blood velocity normally lies between 90 and 140 cm/s (ref 3, p 260). Values significantly above this may indicate local stenosis, while values below can indicate low flow caused by proximal or distal occlusion. The presence of any plaque intruding into the lumen is noted, and the degree of stenosis is estimated. (Figure 8) shows a scan of a femoral artery with a small protruding plaque causing about 20% stenosis.
 Figure 8: A plaque protruding into the lumen of the common femoral artery. The plaque (PL) is small and is causing about 20% stenosis.
Figure 8: A plaque protruding into the lumen of the common femoral artery. The plaque (PL) is small and is causing about 20% stenosis.The shape of the waveform is important because a monophasic waveform can indicate proximal disease in the iliac vessels. The biphasic or triphasic waveform occurs because the main blood vessels in the leg are elastic and are dilated by the increased pressure during systole. This creates a reservoir of blood which empties during diastole. The volume of blood in the reservoir is more than enough to supply the limb, and the excess flows back up the vessel into the abdominal aorta creating the reverse flow component (Figure 9). An abnormal monophasic waveform without the reverse component occurs when the volume in the reservoir is insufficient and extra flow is required during diastole. This is usually because an iliac stenosis or occlusion reduces the blood available to fill the reservoir during systole, but may also occur when there is a large flow to the limb caused by exercise or gross infection.
Proximal disease can also be indicated by turbulence in the common femoral artery. Turbulence is created in eddies distal to a tight stenosis, and the eddies then travel downstream with the blood flow. They can be detected as spikes in the blood velocity waveform (Figure 10), usually on the downslope at the end of systole. The turbulence is more likely to be created during peak systole when the local blood velocity is highest, but appears later than this distally because the systolic pressure pulse travels faster than the eddying blood. The delay between peak systole and the appearance of the turbulence in the common femoral artery can give an indication of the site of the proximal iliac stenosis. Jager et al [7] have described the shape of the waveform in the lower-limb arteries and the changes associated with different degrees of stenosis.
 Figure 9: Schematic generation of a biphasic waveform. During systole (a), the heart pumps blood into the peripheral vessel and the branches. The peripheral vessel expands. During diastole (b), the vessel contracts. There is more than enough blood in the vessel to supply the periphery, and the excess flows backwards into the more proximal branches. A biphasic waveform is obtained at the point marked with an asterisk.
Figure 9: Schematic generation of a biphasic waveform. During systole (a), the heart pumps blood into the peripheral vessel and the branches. The peripheral vessel expands. During diastole (b), the vessel contracts. There is more than enough blood in the vessel to supply the periphery, and the excess flows backwards into the more proximal branches. A biphasic waveform is obtained at the point marked with an asterisk. Figure 10: Turbulence in the common femoral artery. The blood velocity waveform contains spikes (marked I) just after peak systole. These represent turbulence generated from a proximal tight stenosis
Figure 10: Turbulence in the common femoral artery. The blood velocity waveform contains spikes (marked I) just after peak systole. These represent turbulence generated from a proximal tight stenosis  Figure 11: A superficial femoral artery stenosis. The colour image shows aliasing, a sharp transition from maximum speed away from the probe (light blue) to maximum towards (yellow). The lumen is narrower at the stenosis and the brighter echoes in the near wall show the presence of plaque, which is causing shadowing to the right of the image.
Figure 11: A superficial femoral artery stenosis. The colour image shows aliasing, a sharp transition from maximum speed away from the probe (light blue) to maximum towards (yellow). The lumen is narrower at the stenosis and the brighter echoes in the near wall show the presence of plaque, which is causing shadowing to the right of the image. Figure 12: Velocity waveform through a stenosis. The peak blood velocity through this superficial femoral artery stenosis is greater than 3.9 m/s, compared with 0.26 m/s just proximal to the stenosis. This increase in velocity shows a very tight stenosis.
Figure 12: Velocity waveform through a stenosis. The peak blood velocity through this superficial femoral artery stenosis is greater than 3.9 m/s, compared with 0.26 m/s just proximal to the stenosis. This increase in velocity shows a very tight stenosis. If iliac disease is suspected, either from clinical examination or from a monophasic common femoral waveform, the aorto-iliac segment can be scanned [12] . A 3.5 MHz probe is usually used to give better penetration and a wider field of view. Access to the vessels can be difficult if overlying bowel and bowel gas block the ultrasound. Some centres give an enema before scanning this region, but in thin patients a satisfactory examination is often possible without bowel preparation. The operator is again looking for narrowing or blockage of the artery and the same criteria are used. If distal disease is suspected, some of the small arteries below the knee can be examined. The posterior tibial artery is usually accessible just above the ankle from the medial aspect, and can be traced up the leg as it gets deeper. The artery runs parallel to a pair of veins (Figure 13) and this can aid identification. The operator is again looking for evidence of stenosis or occlusion, but these vessels are small and close to the limit of resolution of all but the most modern scanners, and absence of detected flow may indicate lack of sensitivity of the equipment rather than occlusion.
 Figure 13: Peroneal artery and paired veins. The arterial flow, shown in red, is from left to right with a component towards the probe. The venous flow, shown in blue, is in the opposite direction. The anterior and posterior tibial arteries also lie between paired veins.
Figure 13: Peroneal artery and paired veins. The arterial flow, shown in red, is from left to right with a component towards the probe. The venous flow, shown in blue, is in the opposite direction. The anterior and posterior tibial arteries also lie between paired veins.http://www.blogger.com/www.worldwidewounds.com/.../Doppler-Imaging.html
Assessing the lower-limb veins for incompetence
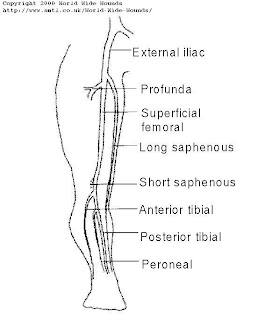 Figure 14: The main veins of the lower limb. The long and short saphenous veins are superficial veins and the remainder are deep veins.
Figure 14: The main veins of the lower limb. The long and short saphenous veins are superficial veins and the remainder are deep veins.
The main reason for scanning these veins is to detect veins in which the valves leak. The leakage may be due to valvular damage or to venous distension [14] . If the valves leak, the veins become incompetent and the blood falls back under gravity as the calf muscles relax, increasing the venous pressure because of the hydrostatic pressure of the column of blood being supported. The condition is known as venous insufficiency. Persistent increased pressure causes superficial veins to dilate producing varicosities, and causing tissue damage distally, showing first as changes in skin colour and progressing to ulceration. The main purpose of treatment is to reduce the excess venous pressure, and this can be done surgically or using bandages. Surgical treatment removes or ties the incompetent veins, and is suitable for superficial vein incompetence since there are other veins which can carry the venous return. The veins are tied at all points where the higher pressure blood from the deep veins enters the superficial system. This is usually at the sapheno-femoral junction or the sapheno-popliteal junction or through incompetent perforators linking the deep and superficial systems. However, the deep veins cannot be removed or tied since they are required to return the blood to the heart. If the deep veins are incompetent, the leg is bandaged to increase the external pressure so that the tissue pressure more closely matches the venous pressure [15] . The applied pressure is graded, being greater at the ankles and decreasing up the leg to encourage the venous return [16] [17] .
The clinical requirement is therefore to identify any superficial incompetent veins and to locate the points where blood is entering from the deep venous system. The presence or absence of deep vein incompetence must also be determined. If there are several incompetent vessels, it can also be useful to indicate which appear to be more significant. A simple examination can be performed using a hand-held continuous wave Doppler unit [18] but this only gives limited information and a full colour duplex scan is preferable. The duplex technique is described in detail by Polak [3]. Patterns of venous reflux have been described by Myers et al [19] and Lees and Lambert [20] , and correlated with clinical symptoms and signs by Labropoulos et al [21] . Further validation of the duplex colour flow examination has been described by Pierik et al [22] .
The examination starts with the patient standing facing the investigator, or lying supine on a couch tilted feet down at least 20� from the horizontal. This is to ensure the veins are filled, and also to ensure that gravity will return blood through any incompetent veins. Coupling gel is put on the probe, which is placed lightly on the skin in the groin over the femoral vein. A light probe pressure is essential, since too great a pressure can narrow or occlude the vein. The femoral vein is identified using the colour Doppler display, and the probe moved along the vein until the site of the sapheno-femoral junction is located (Figure 15). This is near to the point where the femoral vein comes closest to the skin surface. The colour box is placed on the image of the femoral vein just distal to the junction and the thigh is squeezed gently. Flow should be seen in the vein, the colour indicating flow towards the abdomen.The squeeze is then released, and the image inspected for any reverse flow during the release. Any reverse flow persisting for more than one second is normally taken to indicate significant incompetence (ref 3, p 234), although some workers use 0.5 sec [19] or 0.6 sec [20] as the cut-off. The colour box is then placed over the image of the sapheno-femoral junction where the long saphenous vein meets the femoral vein, and the thigh again squeezed and released (Figure 16). Reverse flow persisting for more than 1 sec indicates significant long saphenous vein incompetence.
 Figure 15: Longitudinal scan of a sapheno-femoral junction. The superficial long saphenous vein (LSV) joins the deep superficial femoral vein (SFV) to form the deep common femoral vein (CFV)
Figure 15: Longitudinal scan of a sapheno-femoral junction. The superficial long saphenous vein (LSV) joins the deep superficial femoral vein (SFV) to form the deep common femoral vein (CFV)
 Figure 16: A normal sapheno-femoral junction on squeeze/release. The blue in the long saphenous vein shows flow towards the heart. The blood velocity waveform shows flow towards the heart as the thigh is squeezed and the flow continues in the same direction as the squeeze is released.
Figure 16: A normal sapheno-femoral junction on squeeze/release. The blue in the long saphenous vein shows flow towards the heart. The blood velocity waveform shows flow towards the heart as the thigh is squeezed and the flow continues in the same direction as the squeeze is released.
Although this procedure can seem straightforward, there are conditions which make the study difficult. The thigh can be difficult to squeeze, and in this case a co-operative patient can perform a Valsalva manoeuvre. The patient breathes in, closes their mouth and nose or throat, and increases the abdominal pressure by trying to force air out against the obstruction. The increased pressure is transmitted to the veins, and reverse flow is seen in the femoral or long saphenous veins if these are incompetent.
Some patients may have recurrent incompetence which has developed since previous surgery. In these cases the long saphenous vein (LSV) may have been ligated and may not communicate directly with the femoral vein. However, small collateral veins may have opened, linking the femoral vein to the more distal LSV, or there may be incompetent perforators linking in the same way (Figure 17). The next part of the study is therefore to identify the long saphenous vein at mid-thigh level and to assess the degree of any incompetence in the same way as before. The probe is placed over the long saphenous vein on the antero-medial aspect of the thigh, posterior to the path of the superficial femoral artery. The calf is squeezed and released, and the presence and approximate duration of any reverse flow is noted (Figure 18). If an incompetent LSV is demonstrated, the vein should be traced proximally up the thigh to identify the source of the incompetence and in particular to look for the sites of incompetent perforators, which can be marked on the skin surface with a crayon.
 Figure 17: A large incompetent upper thigh perforator. The large perforator joins the deep superficial femoral vein (SFV) to the superficial long saphenous vein (LSV). On release of a thigh or calf squeeze, blood would flow from the deep vein through the incompetent perforator into the superficial system.
Figure 17: A large incompetent upper thigh perforator. The large perforator joins the deep superficial femoral vein (SFV) to the superficial long saphenous vein (LSV). On release of a thigh or calf squeeze, blood would flow from the deep vein through the incompetent perforator into the superficial system.
 Figure 18: An incompetent long saphenous vein. There is normal forward flow on squeezing the lower thigh (SQ), but the flow reverses when the squeeze is released (REL). The reverse flow persists for more than two seconds, indicating significant incompetence.
Figure 18: An incompetent long saphenous vein. There is normal forward flow on squeezing the lower thigh (SQ), but the flow reverses when the squeeze is released (REL). The reverse flow persists for more than two seconds, indicating significant incompetence.
The patient then turns round to face away from the investigator, and relaxes the leg being examined. The probe is placed behind the knee, the popliteal vein and the sapheno-popliteal junction identified and assessed for incompetence by squeezing and releasing the calf. If superficial incompetence is demonstrated, it is important to identify which vein is incompetent. This is usually the short saphenous vein (SSV), but may also be the posterior thigh vein or the gastrocnemius vein.
Having assessed the main deep and superficial veins, it is important to examine any varicose veins to assess how they are being filled. They are usually filled by reverse flow in an incompetent superficial vein (LSV or SSV) but could be filled directly from incompetent perforators. The probe is placed lightly over the varicose vein, which is then traced up the leg using squeeze/release of the calf or thigh to augment flow and aid identification. Reverse flow on release of the squeeze can usually be seen clearly distally, but this may become harder as the probe is moved up the leg. This is because the varicose vein may be fed by small tortuous incompetent veins which pressurize the system but provide restriction to flow. In this case, squeezing the leg distally forces blood up the leg and this dilates the proximal vein providing a reservoir from which the blood falls back on release of the squeeze. However, more proximally the flow reduces because any reservoir is small or non-existent, and it can be impossible to trace the varicose veins satisfactorily. The veins are often tortuous with many branches and it is important to follow the main channel rather than side branches. The only guide is to try to follow the main incompetent vessel.
At this stage of the examination, a clear picture has hopefully emerged, with the incompetent veins identified and a definite source of the incompetence demonstrated. It is very satisfying when this happens, particularly if the picture is complicated. However, there are times when a satisfactory picture does not emerge, and the report has to reflect this.
The final part of the study is to identify any further incompetent perforators, particularly in the calf. It can be difficult to examine the calf with the patient standing on the floor, so some operators stand patients on a stool. Patients do occasionally become faint and very occasionally collapse without warning, so I prefer to examine the calf with the patient sitting up on a couch with the knee bent so that the calf is about midway between horizontal and vertical. The medial, lateral and posterior aspects of the calf are then scanned in longitudinal mode using the colour Doppler display. Incompetent veins can be identified close to the ankle using squeeze/release of the distal calf, and any incompetent veins tracked up to establish any communication with the deep veins. Sites where blood enters the superficial system on release of the squeeze are marked, and the distances from the lateral or medial malleolus are measured and recorded.
Venous examinations, particularly for recurrent incompetence, can be complex and time-consuming. I generally allow 1 hour for a bilateral scan, and 40 minutes for a unilateral study. More time is required if bandages have to be removed before the study. It is important to make notes of the findings at each stage of the study, and a sketch can sometimes be useful as an aide memoire.
http://www.blogger.com/www.worldwidewounds.com/.../Doppler-Imaging.html
Thursday, July 15, 2010
Cornual pregnancy:

 Sonography of the uterus shows a gestation sac of 6 weeks 4 days age, in the right cornu of the uterus. 3-D image of the uterus further confirms the findings. These ultrasound images are diagnostic of cornual pregnancy (a type of ectopic pregnancy). Ultrasound images courtesy of Dr. Latha Natrajan, Bangalore, India.
Sonography of the uterus shows a gestation sac of 6 weeks 4 days age, in the right cornu of the uterus. 3-D image of the uterus further confirms the findings. These ultrasound images are diagnostic of cornual pregnancy (a type of ectopic pregnancy). Ultrasound images courtesy of Dr. Latha Natrajan, Bangalore, India.Bicornuate uterus with gestation sac:
 Pregnancy in one horn of uterus with two horns (cornu).
Pregnancy in one horn of uterus with two horns (cornu).1=one horn with decidual reaction.
2=another horn with empty pattern.
http://www.ultrasound-images.com/early-pregnancy.htm
Wednesday, July 14, 2010
How to spot the Normal ductus venosus:
 a) First spot the umbilical vein passing through the fetal abdomen.
a) First spot the umbilical vein passing through the fetal abdomen. b) Switch on the color Doppler function to view the flow of the umbilical vein.
b) Switch on the color Doppler function to view the flow of the umbilical vein. c) Reduce or increase the PRF (pulse repetition frequency) function of the color flow until you spot a prominent but short vessel with MARKED ALIASING (ie: turbulent flow producing a multiple shades in the flow image). This is most likely to be the ductus venosus. Note the location of the vessel, just anterior to the fetal aorta.
c) Reduce or increase the PRF (pulse repetition frequency) function of the color flow until you spot a prominent but short vessel with MARKED ALIASING (ie: turbulent flow producing a multiple shades in the flow image). This is most likely to be the ductus venosus. Note the location of the vessel, just anterior to the fetal aorta. d) Now switch on the spectral doppler trace of the vessel. This will give a wavy spectral waveform with 3 waves (d): The S wave, the D wave and the A wave. Note the marked diastolic flow in this waveform. This is diagnostic of a normal Ductus venosus. All images by Joe Antony, MD, using a Toshiba Nemio -XG ultrasound system.
d) Now switch on the spectral doppler trace of the vessel. This will give a wavy spectral waveform with 3 waves (d): The S wave, the D wave and the A wave. Note the marked diastolic flow in this waveform. This is diagnostic of a normal Ductus venosus. All images by Joe Antony, MD, using a Toshiba Nemio -XG ultrasound system. Fetal club hand deformity:



 This 2nd trimester fetus shows short forearm bone (radius) with radial deviation of the wrist. The image 2 nd is a B-mode ultrasound image showing this deformity of the hand. The other 3 images are 3-D (3 dimensional) ultrasound images showing the same anomaly in this fetus. These ultrasound images suggest fetal radial club hand anomaly. Radial club hand is the commonest fetal club hand anomaly. It is important to look for other fetal malformations as this anomaly is usually not isolated. Ultrasound images courtesy of Dr. Dilraj Gandhi, India.
This 2nd trimester fetus shows short forearm bone (radius) with radial deviation of the wrist. The image 2 nd is a B-mode ultrasound image showing this deformity of the hand. The other 3 images are 3-D (3 dimensional) ultrasound images showing the same anomaly in this fetus. These ultrasound images suggest fetal radial club hand anomaly. Radial club hand is the commonest fetal club hand anomaly. It is important to look for other fetal malformations as this anomaly is usually not isolated. Ultrasound images courtesy of Dr. Dilraj Gandhi, India.














